
As we develop new ways of generating electricity, we also need new ways to store it. Grid-scale batteries are necessary to keep power flowing when the sun isn’t shining or wind isn’t blowing. This isn’t just a matter of scaling up the rechargeable lithium-ion batteries found in consumer electronics; entirely new battery technologies are needed to meet the challenges of grid-scale storage.
One promising technology is the iron redox flow battery. Swapping abundant iron for the relatively rare lithium makes these batteries considerably less expensive per kilowatt-hour of electricity, but the water-based mechanism of a flow battery means they quickly degrade. More research is necessary to allow these iron-based batteries to last long enough to be a feasible grid-storage solution.
Tao Gao, assistant professor in the Department of Chemical Engineering, has just received a National Science Foundation CAREER Award to further his work on the fundamental chemistry that governs these reactions.
“Iron is the second most abundant metal in Earth’s crust, and it is the most produced metal in modern society; so a battery based on it is going to be very, very cheap,” Gao says. “But that’s only half of the equation. For it to be worthwhile to build at grid-scale, the battery has to last for a long time, years, or even decades.”
Beyond batteries, a better understanding of iron oxide electro-reduction reactions could unlock entirely new ways of producing metallic iron. Traditional iron production involves smelting ore in highly polluting blast furnaces; electro-reduction could harness renewable energy sources to significantly reduce the carbon emissions from the industry.
“The first step in designing something transformative is understanding the science behind it,” he says. “In this project, we’re also trying to understand how to use electricity to reduce iron ore into metallic iron. Ultimately, that would be a greener method than the status quo.”
The connection between energy storage and metal production is in the chemistry of reducing iron ions or oxides to metallic iron. For the former, reactions must be tailored to keep those iron ions in the solution from dropping out. For the latter, the goal is to efficiently reduce iron oxide in ores at scale while stripping away impurities. But in both cases, a key challenge is to slow down or stop side reactions that get in the way and steer electrons to only reduce iron ions/oxides.
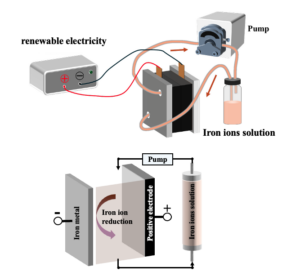
A typical battery consists of three main components: a positive electrode, a negative electrode, and an electrolyte that allows charged ions to travel between them. In an iron redox flow battery, the electrolyte is water with iron ions dissolved within it, which is then “flowed” from a holding tank into a chamber with electrodes on either side, releasing electricity. When there’s excess electricity in the grid, it’s used to pump the spent electrolyte back through the chamber, recharging it on its way to the holding tank so it can repeat the process when energy is needed.
“The problem is that there are two competing reactions going on inside the battery,” Gao says.
“When the electrons reduce water instead of iron ions, the iron ions precipitate out of the solution and can’t be used to store energy anymore. Eventually, the whole battery fails.”
“We’d like to be able to steer the reaction so that the iron reduction uses 99.99% of the electrons and the water reduction uses 0.01% or less,” he says. “This proposal is trying to understand the reactivity of that reaction and how it depends on the properties of the electrolyte, such as its pH, temperature, and chemical composition.”
“Reduction,” in this case, refers to the process of gaining negatively charged electrons. If the water reduction reaction dominates, it releases hydrogen, converts the solution from acidic to basic, forming useless iron hydroxide. Keeping the aqueous solution acidic is also important to the second half of the project: developing new ways of processing low-grade iron ores into iron metal.
“Iron oxide reductions have not been studied much in acidic environments, where you have a lot of reduction of protons, and therefore a lot of hydrogen,” Gao says. “The electricity that drives the reaction would be wasted on that side process, but we found that if you increase ion concentration you can suppress hydrogen production.”
The benefit of reducing iron oxide in an acidic solution is that the acid can help break apart the ore, release iron ions into the solution, and separate impurities that slow down the reaction. With those efficiency gains, electrodeposition-based methods of iron production could eventually surpass carbon-intensive smelting.
Both the energy storage and metal production applications of Gao’s work will require a computational approach to fine-tune the properties of iron-ion-carrying solutions.
“We’re not just doing experiments,” Gao says “we’re building theoretical physics-based models of the reaction to understand it. And with this physical model, we can do computations to predict the behavior of new electrolytes, which can accelerate the design of low-cost iron batteries and green ironmaking processes.”