Behind every “breakthrough” in the fight against cancer is another, slightly less glamorous — but absolutely essential — breakthrough: the awarding of a research grant. Cutting-edge cancer research is inherently expensive and time-consuming. Without the funding of a grant, usually supplied by a governmental or nonprofit organization, a prospective treatment or therapy will never move beyond being just that; a prospective treatment or therapy.
Yet even for the most promising research projects, to secure a grant of millions of dollars is notoriously difficult. With only so much funding to split among the many passionate innovators, granting agencies can only award a small fraction of even the most promising projects.
“The funding rate is very, very low” explains Tom Zangle, Associate Professor of Chemical Engineering and Associate Member of Huntsman Cancer Institute’s Cell Response and Regulation Program. “It’s an incredibly competitive process.”

For the past 10 years, Zangle has been pioneering a technique that uses high-powered, highly-light-sensitive microscopes to quickly test cancer cells’ reaction to different therapies. Leveraging a technology known as quantitative phase imaging, it has the potential to greatly improve patient outcomes by personalizing the type of treatment they receive.
“it’s just a lot faster than traditional methods of testing cancer samples” says Zangle. “And for some of these patients, especially with advanced disease, months really matter.”
Despite their great potential and high scores from grant proposal reviewers, his two current major projects — one focused on treatment-resistant melanoma, the other on advanced metastatic breast cancer — had yet to secure enough funding to proceed to the next phase of research.
“Both projects we submitted multiple times; the melanoma one for a National Institute of Health R01 Grant, and the breast cancer one for an extension of a Department of Defense grant,” Zangle says. “And even though both were right on the cusp, we didn’t make the cut.”
Zangle’s persistence eventually paid off. After submitting each project a fourth time, he received notifications that both had been funded.
“It was a huge surprise!,” he says, smiling. “I was waiting on one or the other project to come in and then they both landed in the span of two weeks.”
The breast cancer project had secured $1.1M in funding over the next three years with Zangle as the sole PI, and the melanoma project $2M over 5 years, to be split among Zangle and his collaborators.
Looking back over Zangle’s career, it’s clear that underlying his lab’s double success is a great deal of persistence.
Although Zangle was not initially interested in biology — his PhD was in mechanical engineering and his early research was focused on the theory of flow in micro- and nanochannels— he was drawn to oncology by its more direct impact on improving people’s lives, as well as a the possibilities raised by an older technology that had recently become much more feasible.
The technology was quantitative phase imaging (QPI) microscopy, first demonstrated on living cells in the 1940’s. Although there are several different QPI methods, they all revolve around shining light through individual cells, measuring aspects of the delayed or refracted light that has passed through the cells, and then using that data to study the cells’ behavior. This technique is especially helpful in tracking changes in a cell’s mass over time.
“Effectively, it’s using light to weigh cells,” Zangle explains.
Unfortunately for scientists in the mid twentieth century, not only was it difficult to reach the required level of precision and sensitivity, but the resulting sea of data was too much to manually process and analyze.
“The early techniques to do QPI were very laborious, very time consuming to generate and process the data. It languished for decades as a technique in biology,” says Zangle.
By the time he finished pursuing a PhD at Stanford, however, advances in imaging, photonics, computing, and the technology itself had finally opened the doors for use in biological research.
“So I jumped in,” he recalls.
Since there weren’t many papers applying QPI to biology, Zangle and his collaborators were in uncharted territory. Tracking the changes in cells over time, for example, still had to be done by hand. Sensing a need, Zangle used his postdoc at UCLA to develop an analytical software system that automated the cell tracking process.
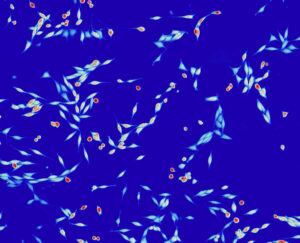
During that time he was working in a molecular biology lab, continuing to experiment with and configure his cell imaging systems, with mixed results.
“We did some early experiments on patient samples,” he laughs, “and they completely failed.”
It wasn’t until Zangle arrived at the University of Utah in 2016 and started working with the Huntsman Cancer Institute that this area of his research really began to gain traction.
“I was able to get some experiments to work that I’d wanted to work for years,” he says, “it was really a matter of having the right samples and the right collaborators.”
One of those collaborators was the Huntsman Cancer Institute’s Robert Judson-Torres, assistant professor of dermatology and adjunct assistant professor of oncological sciences in the School of Medicine. Judson-Torres had been working with QPI and other techniques to learn about melanoma cell changes over time and in response to therapy, so Zangle’s ability to analyze and process QPI data made teaming up an obvious choice. It was their combined conviction in the potential of their work that convinced the NIH funding council to finally award them the R01 grant.
The final thing that helped the breast cancer project receive funding was, Zangle believes, connecting with former patients.
“Something that’s been really nice is that the Huntsman Cancer Institute has a very involved patient advocate team,” he says. “These are women who are survivors of breast cancer and volunteer with Huntsman researchers and clinicians to help them understand where patients are coming from and what patients would need or want.”
“Throughout this project, I’ve had a chance to talk with those patients, hearing their stories and how this technique could have helped with their own course of treatment. It’s really inspiring to see their enthusiastic response to our work.”
So enthusiastic in fact, that the patient advocate committee offered to write a letter of support to submit with the breast cancer grant. Zangle thinks it was this, along with their input on the project’s abstract and overall framing, that ultimately helped the project finally receive funding.
Now with two grants in hand, Zangle looks forward to, if everything goes as planned, make critical steps towards clinical trials, and then on to real world application.
“I’ve worked on this for over a decade but there’s still a lot more to go before it turns into something that helps patients.”